
Insights+: EMA Marketing Authorization of New Drugs in August 2024
Shots:
-
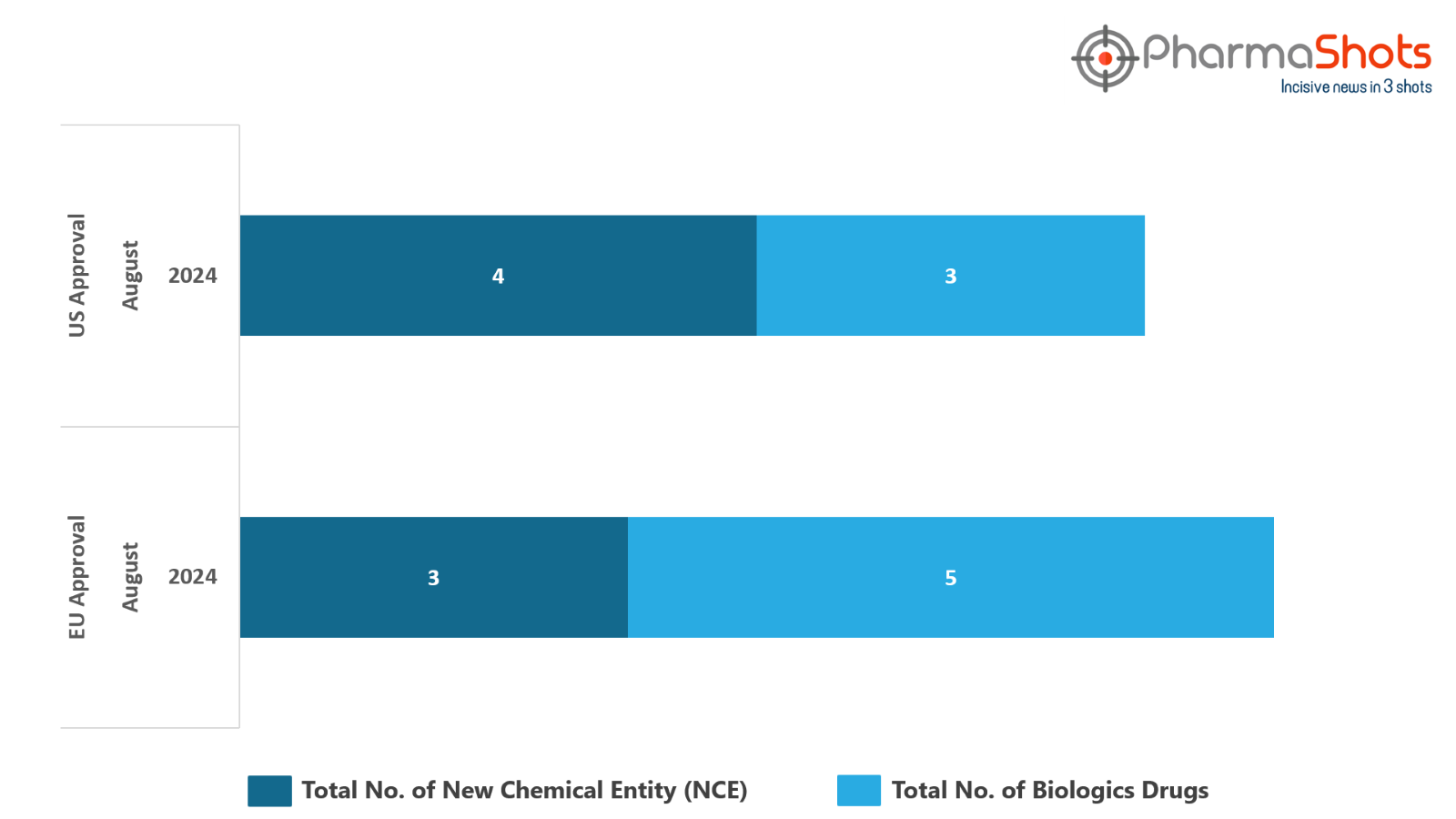
The EC has approved to 5 Biologics and 3 New Chemical Entities in August 2024, leading to treatments for patients and advances in the healthcare industry
-
The major highlighted drugs were Johnson & Johnson’s Balversa to treat Metastatic Urothelial Carcinoma (mUC) and Takeda’s Adzynma for Congenital Thrombotic Thrombocytopenic Purpura (cTTP)
-
PharmaShots has compiled a list of 8 drugs that have been approved by the EC
Product Name: Adzynma
Active ingredient: Recombinant ADAMTS13
Company: Takeda
Date: Aug 01, 2024
Disease: Congenital Thrombotic Thrombocytopenic Purpura (cTTP)
Shots:
-
The EC has approved Adzynma (recombinant ADAMTS13) to treat ADAMTS13 deficiency in cTTP adults & children. Adzynma is also being assessed under P-IIb study for immune-mediated thrombotic thrombocytopenic purpura (iTTP)
-
Approval was based on the P-III study assessing Adzynma to treat cTTP, published in the NEJM, with patients receiving Adzynma (40IU/kg, IV, weekly) or plasma-based therapy for mos.1-6 (period 1), switched treatments for mos.7-12 (period 2) & then Adzynma for mos.13-18 (period 3)
-
Study depicted 0 (Adzynma) vs 1 (plasma-based therapy) acute TTP event and 1 vs 7 (in 6 patients) subacute TTP events in periods 1 & 2. Efficacy data from period 3 was consistent with periods 1 & 2
Product Name: mRESVIA
Active ingredient: mRNA-1345
Company: Moderna
Date: Aug 22, 2024
Disease: Lower Respiratory Tract Disease (LTRD)
Shots:
-
The EC’s approval of mRESVIA (mRNA-1345) vaccine to prevent LTRD due to RSV infection among adults was supported by P-III (ConquerRSV) trial in adults (n=37,000; ≥60yrs.) & is valid across EU plus as Iceland, Liechtenstein & Norway
-
Primary analysis (3.7mos. median follow-up) depicted vaccine efficacy (VE) of 83.7%, published in the NEJM. Supplementary analysis (8.6mos. median follow-up) showed sustained VE of 63.3% against RSV-LRTD incl. ≥2 symptoms with VE of 74.6% & 63% with ≥2 & ≥3 symptoms, respectively
-
In addition, the vaccine received the US FDA’s approval in May 2024 for the same. The company has filed MAA to other global authorities
Product Name: Eurneffy
Active ingredient: Epinephrine
Company: ARS Pharmaceuticals
Date: Aug 22, 2024
Disease: Type I Allergic Reactions (Anaphylaxis)
Shots:
-
Following the US FDA approval, the EC has approved Eurneffy (2mg) for type I allergic reactions (anaphylaxis) in adults & children (≥30kg). It will be available in Q4’24 through a pharmaceutical company
-
The approval was supported by the results from a study, involving ~1200 administrations among >700 subjects, along with studies and peer-reviewed literature supporting these results
-
The study assessed PK/PD of Eureffy (2mg) under various conditions such as single and repeat dosing, self-administration, pediatric dosing & in nasal conditions like congestion and rhinorrhea due to allergens or infections like cold/flu
4. Roche’s PiaSky Receives the EC’s Approval to Treat Paroxysmal Nocturnal Haemoglobinuria (PNH)
Product Name: PiaSky
Active ingredient: Crovalimab
Company: Roche
Date: Aug 22, 2024
Disease: Paroxysmal Nocturnal Hemoglobinuria (PNH)
Shots:
-
The EC has granted approval to PiaSky (crovalimab) for treating PNH in adults & adolescents (≥12yrs., weight: ≥40kg) who are either treatment-experienced or treatment-naïve
-
Approval was based on P-III (COMMODORE 2) trial assessing PiaSky vs eculizumab in PNH patients not treated with C5 inhibitors plus results from another P-III trials, COMMODORE 1 (PNH patients switching from C5 inhibitors) & COMMODORE 3 (new to C5 inhibitor treatment in China)
-
COMMODORE 2 showed PiaSky (SC, Q4W) controlled disease, was well-tolerated & non-inferior to eculizumab (C5 inhibitor, given IV, Q2W) with similar safety profiles & AE rates
5. Johnson & Johnson’s Balversa (Erdafitinib) Receives the EC’s Approval to Treat Urothelial Carcinoma
Product Name: Balversa
Active ingredient: Erdafitinib
Company: Johnson & Johnson
Date: Aug 22, 2024
Disease: Metastatic Urothelial Carcinoma (mUC)
Shots:
-
The EC has approved Balversa (oral, QD) monotx. for treatment-experienced adults with inoperable or metastatic urothelial carcinoma (mUC) having susceptible FGFR3 genetic alterations
-
Approval was supported by results of cohort 1 from the P-III (THOR) trial assessing the safety & effectiveness of Balversa (n=136) vs CT (n=130) to treat mUC with select FGFR alterations and has progressed post previous treatments
-
In Jun 2023, the THOR study was stopped early due to positive interim results, allowing CT patients to switch to Balversa. Erdafitinib showed mOS of 12.1mos. vs 7.8mos. & mPFS of 5.6mos vs 2.7mos., with a confirmed ORR of 35.3% vs 8.5%
Product Name: Winrevair
Active ingredient: Sotatercept
Company: Merck
Date: Aug 26, 2024
Disease: Pulmonary Arterial Hypertension (PAH)
Shots:
-
The EC has granted approval to Winrevair (45 & 60mg) combined with other PAH therapies to treat PAH, valid across whole EU as well as Iceland, Liechtenstein & Norway
-
Approval was based on the P-III (STELLAR) study evaluating the safety & efficacy of Winrevair (target dose 0.7mg/kg, SC, Q3W; n=163) vs PBO (n=160) + stable background therapy in PAH patients (N=323)
-
Winrevair improved the 1EP of 6-minute walk distance of 40.8 meters at 24wks. as well as 2EPs of reducing the death risk from any cause & PAH clinical worsening by 82% (number of events: 7 vs 29)
Product Name: Ordspono
Active ingredient: Odronextamab
Company: Regeneron
Date: Aug 26, 2024
Disease: Follicular Lymphoma & Diffuse Large B-cell Lymphoma (FL & DLBCL)
Shots:
-
The EC has approved Ordspono for treating r/r FL or r/r DLBCL in patients who have progressed after ≥2L of systemic therapy
-
Approval was supported by Ordspono’s P-I (ELM-1; n=60) trial in patients with CD20+ B-cell malignancies, incl. those who progressed post CAR-T therapy & P-II (ELM-2; n=128) trial for 5 B-cell lymphoma subtypes such as DLBCL, FL, mantle cell lymphoma, marginal zone lymphoma
-
R/R FL patients (ELM-2) had an ORR of 80% & CR in 73% (mDoR: 25mos.); r/r DLBCL patients naïve to CAR T therapy (ELM-2) had an ORR of 52% & CR in 31% (mDoR: 18mos.); r/r DLBCL patients who progressed post CAR-T therapy (ELM-1) had an ORR of 48% & CR in 32% (mDoR: 15mos.
Product Name: Akantior
Active ingredient: Polihexanide
Company: SIFI and Avanzanite Bioscience
Date: Aug 27, 2024
Disease: Acanthamoeba Keratitis (AK)
Shots:
-
Following recommendations from the EMA’s CHMP & COMP, the EC has approved Akantior to treat AK among adults & adolescents (≥12yrs.)
-
Approval was based on the data from P-III (ODAK) study in AK patients (n=135), published in Ophthalmology, demonstrating that the disease cured in 84.8%, full vision restoration was found in 66.7% with none of them requiring optical cornea transplant and 7.5% needed a therapeutic cornea transplant
-
Avanzanite received the exclusive commercialization rights of Akantior in European Economic Area and Switzerland as per an agreement b/w the company and SIFI
Related Post: Insights+: EMA Marketing Authorization of New Drugs in July 2024
Tags

A passionate content writer with expertise in delivering high-quality and engaging content, Dipanshu is a keen reader and a versatile writer. Dipanshu dedicatedly covers news ranging from biopharma, life sciences, biotech, and MedTech to diagnostics and animal health companies, FDA, EMA, and biosimilar approvals. He can be contacted at connect@pharmashots.com














